
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
Grade: USP/EP
Assay: ≥99.0%
CAS No.: 182410-00-0
Appearance: White to off-white powder
Applications: Injectable and parenteral drug formulations, solubilizer and stabilizer for poorly soluble APIs
Packaging: 500 g/bag; 10 kg/drum or customized packaging available
Free Sample: 100 g available for evaluation
Shipping: Global shipping available
Features: High water solubility, excellent safety profile, strong inclusion complex formation
DELI is a leading manufacturer and exporter of DMF Betadex Sulfobutyl Ether Sodium 182410-00-0 in China. Our product DMF number is 034773. Betadex Sulfobutyl Ether Sodium Injection Grade is a chemically modified beta-cyclodextrin used as drug delivery system. It is applied to injectable drugs to improve the solubility of difficult soluble drugs, can make the drug injections in the rapid transfer and reach the required dosage, reduce irritation to the parts injected, improve the stability of the drug solution and to improve the safety of medication.
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 has been used in injection, oral, nasal and eye medicines. It has a special affinity and inclusion for nitrogen drugs. Increase the drug's stability, solubility, safety (Include drug molecules to form non-covalent). Reduce renal toxicity, ease drug hemolysis. Control the drug release rate, cover the bad smell. Our company produces Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 and Hydroxypropyl Βetadex CAS 128446-35-5 all can provide free samples.


1. Reply to your inquiry promptly and deliver the goods promptly.
2. The company's product Hydroxypropyl Betadex quality assurance and stable supply, it meets the standard requirements of ChP, USP and EP. Betadex Sulfobutyl Ether Sodium Injection Grade meets the standard requirements of USP and EP.
3. We can customize products as your special requirement.
4. Betadex Sulfobutyl Ether Sodium Injection Grade is exported to Asia, Europe, North America, Africa and other countries.

Product FAQ
Q1: Is Xi’an Deli Biochemical a manufacturer of SBECD?
A: Yes, Xi’an Deli Biochemical is a professional manufacturer of cyclodextrin derivatives, including SBECD and HPBCD.
Q2: Can I get free samples for evaluation?
A: Yes, free samples are available for evaluation.
Q3: What is the batch production and annual capacity of SBECD?
A: SBECD batch production is approximately 2.5 tons, and annual production exceeds 200 tons, ensuring stable supply.
Q4: What are the shipping and packaging options?
A: Standard packaging is 500 g/bag or 10 kg/drum. Global shipping is available via air, sea, or express courier.
Q5: What regulatory support is provided?
A: COA, MSDS, stability data, and technical dossiers are available for all batches.

 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0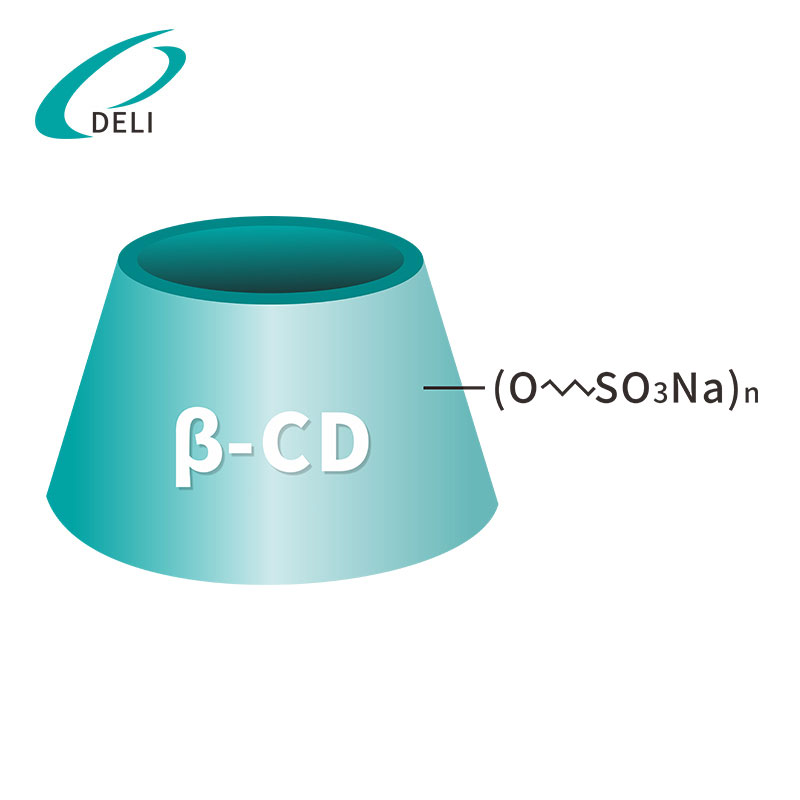 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations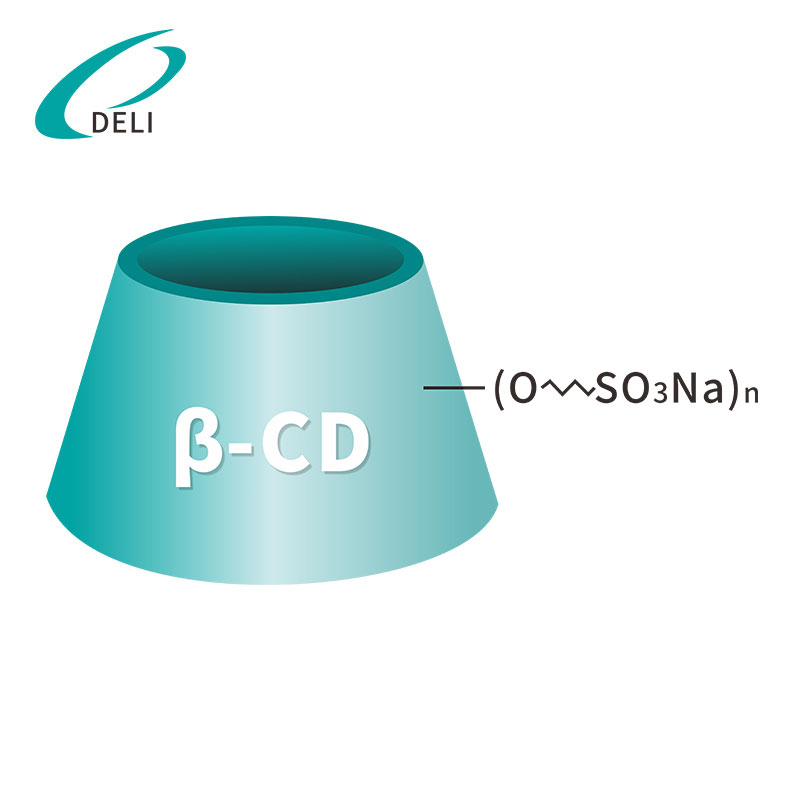 Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 Sulfobutyl beta cyclodextrin 182410-00-0
Sulfobutyl beta cyclodextrin 182410-00-0